Bactorinol® Nasal Drops: A Study Confirms Its Potential Against Chronic RhinoSinusitis
Bactorinol® is a medical device available as nasal drops, formulated with Winterized Lentisk Oil (WLO) derived from Pistacia Lentiscus berries. This unique ingredient has been shown to reduce biofilm formation and alleviate Chronic RhinoSinusitis (CRS) symptoms.
Recent research further supports its efficacy, demonstrating significant improvements in CRS outcomes, including a substantial reduction in biofilm presence and related symptoms.
Bacterial Infections, Biofilms, and CRS
Chronic rhinosinusitis (CRS) is a prevalent and persistent condition that significantly impacts patients’ quality of life, leading to recurrent medical consultations and antibiotic use. Although viral infections often initiate the condition, bacterial infections, particularly those involving biofilm formation, frequently complicate the disease. Common pathogens include Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis.
The development of bacterial biofilms—a complex matrix of polysaccharides that shield bacteria from antibiotics—poses a significant challenge. This biofilm formation contributes to the persistence and recurrence of CRS, making it difficult to eradicate with standard antibiotic therapies. The growing concern of antibiotic resistance further underscores the need for alternative or complementary treatments that can effectively disrupt biofilms and enhance bacterial clearance.
Winterized Pistacia Lentisk Oil: A Potential Game-Changer
A recent study titled “The Role of Pistacia Lentiscus in the Prevention of Chronic Rhinosinusitis Recurrence” , published in January 2024, explored the antibacterial efficacy of Winterized Lentisk Oil (WLO) from Pistacia Lentiscus berries in treating CRS. This study, led by Prof. Macchi, investigated the potential of WLO to disrupt biofilms and reduce the recurrence of CRS in patients.
Lentisk, a Mediterranean shrub, has been used in traditional medicine for centuries. Its oil contains anacardic acids, which exhibit significant antibacterial properties. Through a refining process called winterization, these acids are concentrated, enhancing their biofilm-disrupting capabilities. The study evaluated WLO’s effectiveness in reducing biofilm-associated bacteria and improving clinical outcomes in CRS patients.
Study Findings: Bactorinol® Nasal Drops' Impact on CRS
The clinical trial involved 100 patients with Chronic RhinoSinusitis, with an average age of 46 years, experiencing approximately five CRS episodes annually. Patients were randomized into two groups:
- Group 1: 50 patients treated with isotonic saline nasal irrigation plus 5 drops per nostril of Bactorinol® twice daily for 30 days.
- Group 2: 50 patients treated with isotonic saline nasal irrigation plus 5 drops per nostril of a placebo twice daily for 30 days.
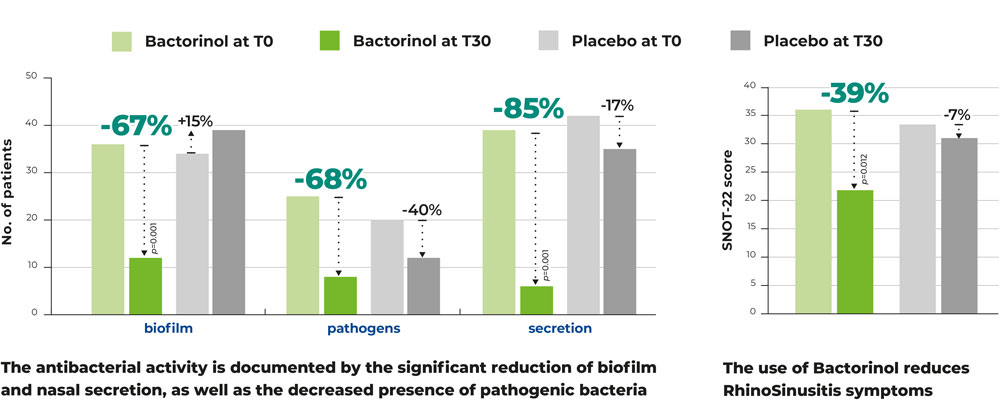
The study’s primary endpoints included the Sino-Nasal Outcome Test (SNOT-22) scores to assess the impact of CRS on quality of life, nasal symptoms, and psychological well-being. Additional endpoints were nasal secretion levels, ciliary motility, bacterial biofilm presence (measured through staining methods), and ciliated cell integrity (measured by the supranuclear stria index).
After 30 days of treatment, the results in the Bactorinol® group were compelling:
- -67% decrease in bacterial biofilm presence.
- -68% reduction in pathogenic bacteria.
- -85% decrease in nasal secretions.
- -39% reduction in rhinosinusitis symptoms, as measured by the SNOT-22 score.
- Improved ciliary motility and enhanced integrity of ciliated cells.
These findings highlight Bactorinol® nasal drops as a promising solution for managing CRS and add further evidence to the already existing research on Winterized Lentisk Oil and its ability to disrupt bacterial biofilm.
Bactorinol® Nasal Drops: A Revolutionary Approach to CRS Management
By disrupting bacterial biofilms and reducing pathogenic bacteria, Bactorinol® not only alleviates symptoms but also addresses the root causes of chronic infection, potentially reducing the need for repeated antibiotic courses and minimizing the risk of antibiotic resistance.
The oily formulation of Bactorinol® ensures prolonged contact with the nasal mucosa, allowing for sustained antibacterial activity and mucus clearance. This makes it a candidate adjunctive therapy for patients with chronic rhinosinusitis, offering hope for those struggling with persistent and recurrent symptoms.
In conclusion, Bactorinol® nasal drops, containing Winterized Lentisk Oil, represent a novel and effective approach to combating chronic rhinosinusitis, improving patient outcomes and enhancing quality of life.
Bactorinol® nasal drops was shortlisted as one of three finalists in the Product of the Year: Botanical category at the NutraIngredients Awards 2023
A formal application for certification under European Regulation (EU) 2017/745 (MDR) for Bactorinol® as a medical device has already been submitted to ensure its future commercialization remains possible.